What Sublevel Immediately Follows the 4s Sublevel
To check your complete electron configuration look to see whether the location of the last electron added corresponds to the elements position on the periodic table. Models of the Atom and periodic Trends Exam Study Guide 7 74.

Electron Arrangement Ck 12 Foundation
2s 2p 3s and so onThe Aufbau principle states that an electron occupies orbitals.
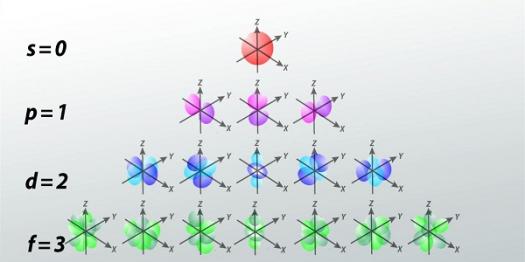
. How many orbitals does an f sublevel have. Equally its requested how many orbitals are in the N 3 degree. The third principal power degree has three sublevels sp and dThe sublevels have varied numbers of orbitals which are areas of likelihood of discovering an electron and every orbital can have a most of two electrons.
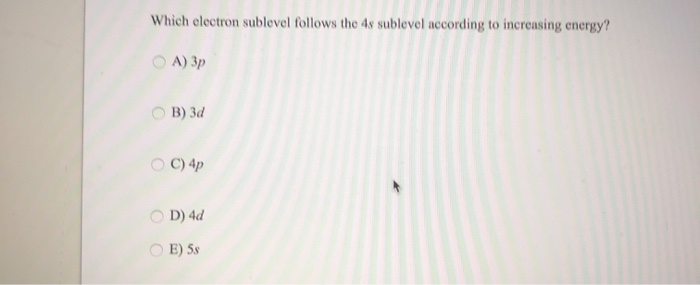
What are the sublevels in order. The 4d sublevel is filled next after the 5s sublevel. Which electron sublevel follows the 4s sublevel according to increasing energy.
Electrons fill energy levels and sublevels _____ in energy first. The Aufbau principle is mostly used in chemistry and it should show us a prediction of the electronic configuration of atom. A 3d B 4s C 4p D 4d E 5s.
Who are the experts. A 3p B 3d C 4p D 4d E 5s. 2p will be filled next with the maximum of 6 electrons.
Qa 200 - 4P. Since 4s sublevel is lower in energy than the 3d sublevel electrons will be added to the 4s first followed by 3d. The 4s sublevel fills before the 3d sublevel begins to fill with electrons because the 3d sublevel has higher energy than the 4s sublevel.
Electrons occupy orbitals in a definite sequence filling orbitals with lower energies first. What is the price elasticity of demand if the price of artichokes is 10. Asked Sep 12 2016 in.
We review their content and use your feedback to keep the. These sublevels hold different amounts of electrons. What are the orbitals that make up the n1 energy level.
How many electrons can an d sublevel hold. But in the third level the energy ranges of the principal. Chemistry questions and answers.
Which electron sublevel follows the 3d sublevel according to increasing energy. Aufbau principle was discovered in the early 1920s and depicts that in the ground state of an atom electrons fill atomic orbitals in order of their increase in energy. A 3s B 3p C 4s D 4p E 4d.
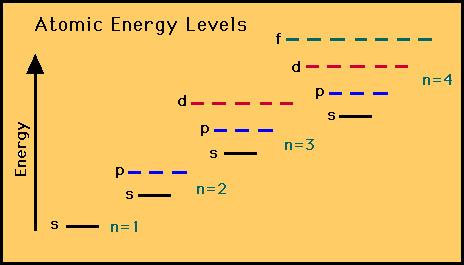
So we fill in 4s next then go back. Subtract 025 cm from 115 cm and round off the answer. 1s 2s 2p 3s 3p now next is 3d BUT the d orbitals are complex and rather high in energy so actually 4s is lower in energy.
The lowest energy sublevel is always the 1s sublevel which consists of one orbital. 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 1s will be filled first with the maximum of 2 electrons. Which electron sublevel follows the 4s sublevel according to increasing energy.
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s 2 5f14 6d10 7p6 Source. Which electron sublevel follows the 4s sublevel according to increasing energy. Add electrons to the sublevels in the correct order of filling.
We fill in electrons according to lowest energy sublevels first. How many electrons can the first energy level hold. Which electron sublevel immediately follows the 4p sublevel according to.
Electrons fill the sub levels in energy order. What is Electron configuration. Which of the following statements is true about the 3s and the 4s sublevels.
This means that the atomic orbitals with the lowest energy are filled by the electrons before occupying the upper atomic orbital. Experts are tested by Chegg as specialists in their subject area. The order in which the atomic orbitals are.
This problem has been solved. Generally orbitals in a lower energy level have lower energies than those in a higher energy level. Suppose that the demand for artichokes Qa is given as.
Which electron sublevel immediately follows the 4s sublevel according to increasing energy. For 4s4p3d the nl value is 404415325 respectively. Asked Sep 12 2016 in Chemistry by Roxxy.
Challenge your knowledge and complete this quiz. What is the term for a property that cannot be observed without changing the chemical formula of a substance. These sublevels have the same shape.
Energy is directly proportional to nl value. Of the following which sublevel is filled last. One can also ask what are the doable sublevels for N 3.
Which electron sublevel follows the 4s sublevel according to increasing energy. 5 Ionization energy is the amount of energy required to release an electron from the outermost. The maximum number of electrons that can fit into the 2nd energy level is.
2s will be filled next with the maximum of 2 electrons. Of the following which sublevel is bartleby. Which has more energy 4s or 4p.
Add two electrons to each s sublevel 6 to each p sublevel 10 to each d sublevel and 14 to each f sublevel. The 3d sublevel is not filled until after the 4s sublevel because the 3d sublevel has more energy than the 4s sublevel and less energy than the 4p sublevel. Create your own Quiz.
4 Electrons are filled into respective orbitals based on the Aufbau rule according to which lower energy levels will fill up prior to those in the higher energies. How many atomic orbitals are there in the d sublevel. How many f orbitals can an f sublevel hold.
Asked Sep 12 2016 in Chemistry by mlj15. These sublevels are the same distance from the nucleus. As we proceed with atoms with multiple electrons those electrons are added to the next lowest sublevel.
Write the Electron configuration for Germanium atom. Why does an electron occupy the 4s. Which electron sublevel follows the 4s sublevel according to increasing energy.
So we basically go in order. The single electron of the hydrogen atom will occupy the 1s orbital when the atom is in its ground state. The second energy level n2.
Explain why the 4s sublevel fills before the 3d begins to fill as electrons are added. Which electron sublevel immediately follows the 3d sublevel according to increasing energy. What is the correct order of increasing energy.
Thus 4s has the least energy. 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p If we add the number of electrons that each sublevel holds it looks like this. Which electron sublevel follows the 3d sublevel according to increasing energy.
The sequence of orbital energy levels is as always-1s 2s 2p 3s 3p 3d.
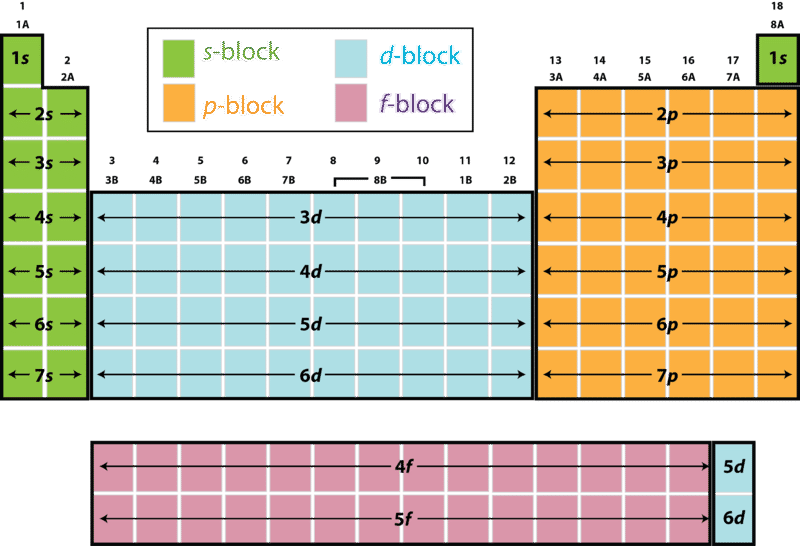
Electron Configuration And The Periodic Table Ck 12 Foundation

Solved Chapter 4 Problem 74e Solution Introductory Chemistry 7th Edition Chegg Com

Example Exercise 4 1 Atomic Notation Ppt Download

Do You Know Aufbau Principle Proprofs Quiz

Aufbau Principle Flashcards Quizlet
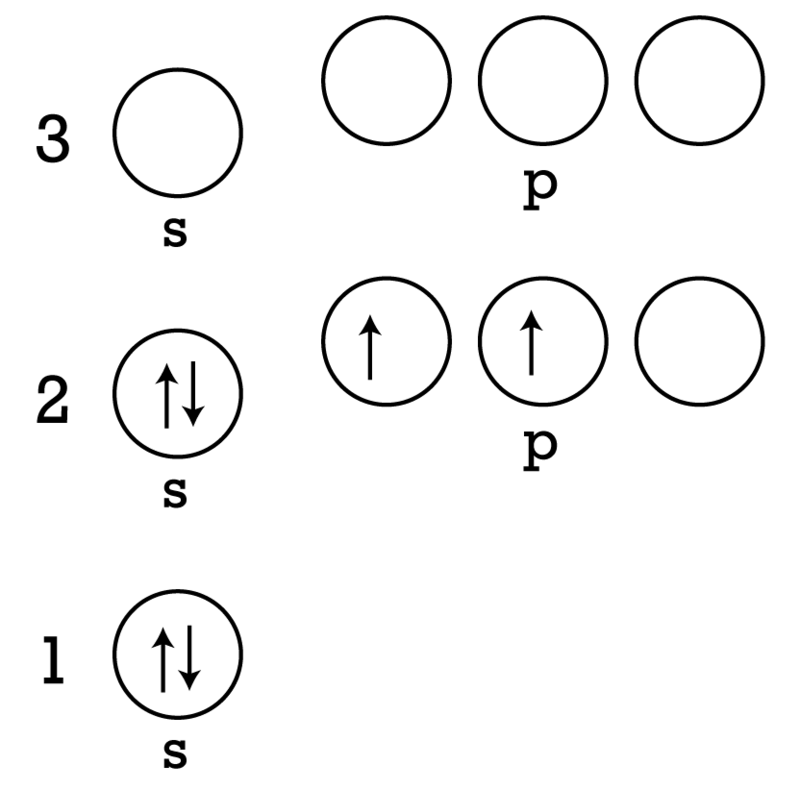
Electron Arrangement Ck 12 Foundation
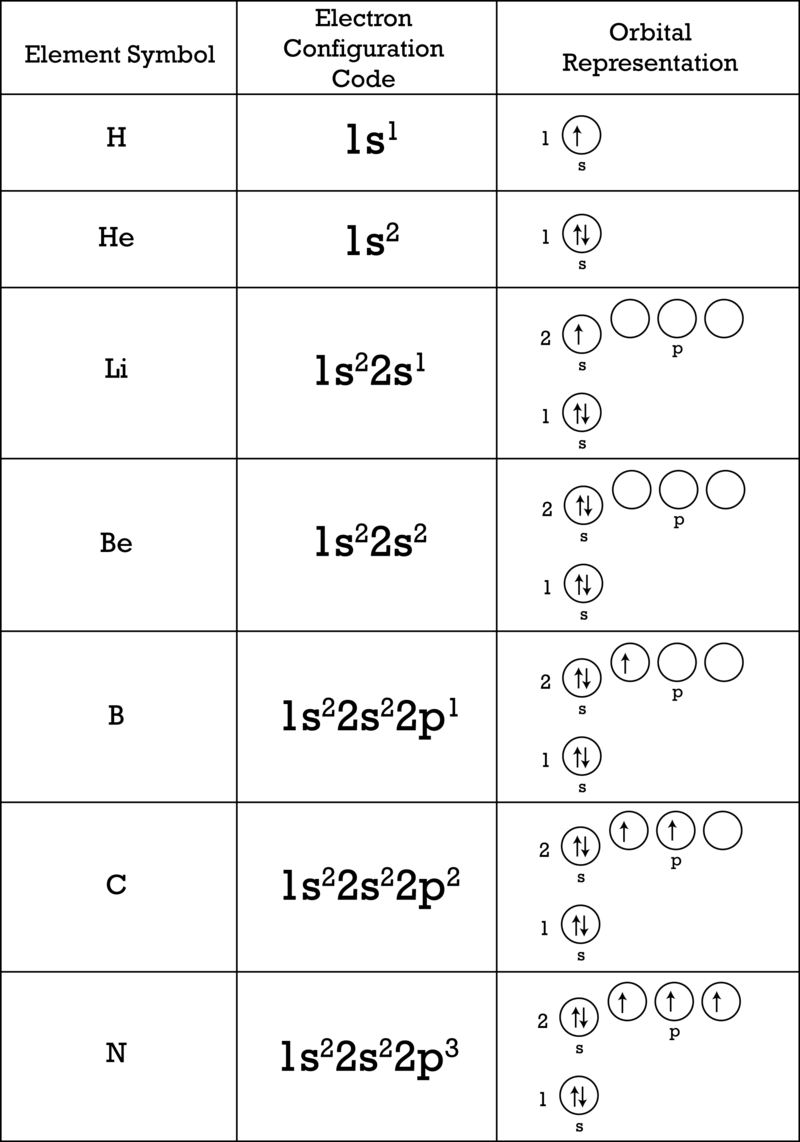
Electron Arrangement Ck 12 Foundation

If 4s Orbitals Are Higher In Energy Than 3d Orbitals Then Why Do Electrons Fill Up In 4s Before Filling Up In 3d Quora

Aufbau Principle Ck 12 Foundation

Example Exercise 5 1 Atomic Notation Ppt Download
Do You Know Aufbau Principle Proprofs Quiz
Do You Know Aufbau Principle Proprofs Quiz

Do You Know Aufbau Principle Proprofs Quiz
Do You Know Aufbau Principle Proprofs Quiz
Solved Which Electron Sublevel Follows The 4s Sublevel Chegg Com
If 4s Orbitals Are Higher In Energy Than 3d Orbitals Then Why Do Electrons Fill Up In 4s Before Filling Up In 3d Quora

Do You Know Aufbau Principle Proprofs Quiz

Comments
Post a Comment